Introduction
Randomised trials are recognised as the gold standard to test interventions to improve outcomes of patients with acute myeloid leukaemia (AML). However, there is a pressing need to understand outcomes in non-selected patient cohorts from real-world datasets. We report outcomes of 1- and 5- year overall survival (OS) following AML diagnosis and 30-day mortality after systemic anti-cancer therapy (SACT) using national cancer registry data in England of the United Kingdom.
Methods
The National Cancer Registration Dataset (NCRD) and the Systemic Anti-Cancer Therapy Dataset (SACT dataset) were used. The NCRD holds the population-based national cancer registry for England (Henson el. Al. Int J Epidemiol. 2020;49:16-16h). The SACT dataset is a population-based resource of treatments delivered in secondary and tertiary settings (Bright el. al. Int J Epidemiol. 2020;49:15-15l).
Patients aged 18-99 years and diagnosed with AML between 01.01.2013 and 31.12.2020 were included and identified in NCRD using ICD-O3 coding (Fritz et. al. WHO 2013). Patients recorded as receiving SACT ≤90 days after diagnosis with a regimen typically used for AML were extracted using the SACT dataset up to 31.12.2021. Survival was followed up to 31.12.2022.
OS with 95% confidence interval (95% CI) following AML diagnosis were calculated using Kaplan-Meier methodology. Hazard ratios (HRs) with 95% CI were generated using Cox proportional hazards regression to assess how covariates (intensity of SACT, age at diagnosis, gender, ethnicity, Index of Multiple Derivation (IMD), comorbidity, and year of diagnosis) were associated with 1- and 5-year survival. 30-day mortality after the initial SACT was calculated for the treated patients. Odds ratios (ORs) with 95% CI were generated using logistic regression to examine how covariates listed above, plus ECOG score of performance status (PS) and body mass index (BMI) were associated with the likelihood of dying ≤30 days.
Results
17,107 patients were identified, of whom 7,906 (46%) had documented SACT. The median age at diagnosis was 72 years, (66 years and 77 years with and without documented SACT).
Median survival following AML diagnosis was 0.6 years (95%CI: 0.5-0.6). 1- and 5-year OS were 39.8% (95% CI, 39.1-40.5) and 18.8% (95% CI, 18.1-19.4), respectively. Survival varied by intensity of SACT, patients received intensive SACT (IT) had a significantly better chance of surviving at 1- and 5-year than patients received non-intensive SACT (NIT) or without documented SACT (Table 1). Survival decreased with increasing age. Patients of Asian and Black ethnicity had a better survival outcome than White. Patients from the least deprived areas had improved 1- and 5-year survival versus those from the most deprived areas. People with no comorbidity had a better survival than those with comorbidities. There was no significant difference in survival by gender. Patients diagnosed in 2013 and 2020 had a significant (p<0.05) 1-year adjusted HR when compared to patients diagnosed in 2017, but their 5-year adjusted HRs were non-significant. This is very likely due to data issues as 2013 was the year cases in the NCRD began to be coded into ICD-O3 codes and 2020 was the year when a national lockdown due to COVID-19 took place.
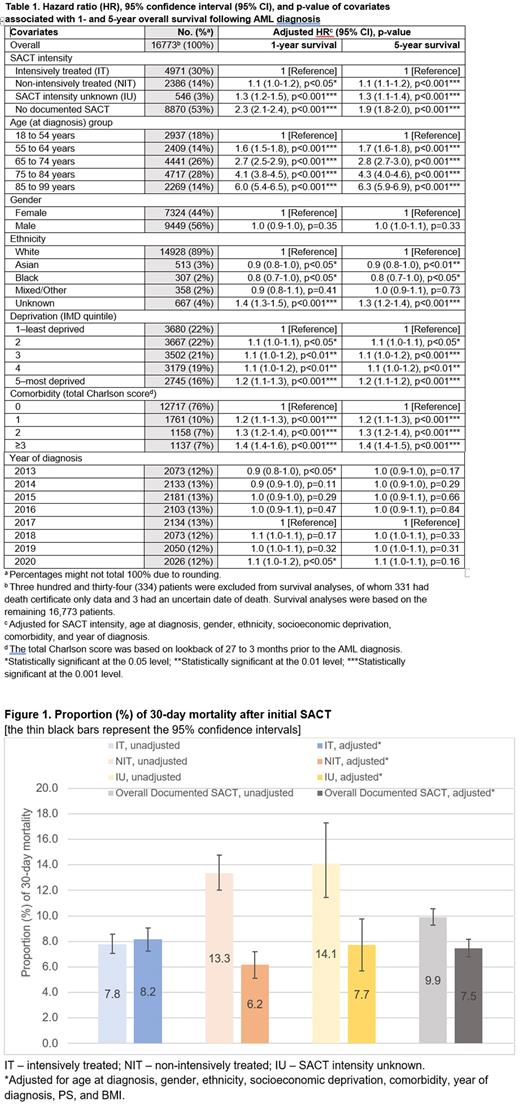
Unadjusted 30-day mortality for IT and NIT was 7.8% and 13.3%, respectively (Figure 1). After adjusting for covariates, patients who received NIT were less likely to die ≤30 days than IT patients (adjusted OR, 0.7, 95% CI, 0.6-0.9 p<0.01). Older patients, those from most deprived areas and those with worse PS all had higher 30-day mortality. Gender, known ethnicity, comorbidity, year of diagnosis, and known BMI were not significantly associated with 30-day death after SACT.
Conclusions
This study illustrates the value of large real-world data sets. Our data confirmed known negative associations of older age, comorbidity, and poor PS on outcomes. We demonstrate that, in England, white ethnicity is associated with reduced survival after accounting for covariates. Socioeconomic deprivation was negatively associated with both OS and 30-day mortality. Further work is needed to understand whether disease biology or other factors are responsible for this disparity. Our study underlines the importance of careful selection of treatment intensity given the increased adjusted 30-day mortality from intensive treatment.
Disclosures
No relevant conflicts of interest to declare.